What is a Battery C Rating: Explore the Mystery of Battery C Rating

What Is Battery C Rating?
The battery C rating indicates how quickly a battery can be charged or discharged relative to its maximum capacity. It defines the current at which energy is delivered or received and serves as a guideline to ensure the process happens safely and efficiently without harming the battery’s performance.
Typically, a battery’s capacity is rated and labeled based on a 1C rate (1C current), meaning that a fully charged 10Ah battery should be able to deliver 10 amps of current for one hour. For example, if a 10Ah battery is discharged at a 0.5C rate, it will supply 5 amps of current for two hours; if discharged at a 2C rate, it will provide 20 amps of current for 30 minutes.
In this article, we will explore a series of issues such as the influencing factors and calculation methods of battery C rating.
Factors Affecting Different C Ratings of Batteries
In many applications, we want the battery to charge quickly rather than waiting for a long time, which involves the battery's C rate. So, what factors influence the battery's rated power?
1. Thermal Heating Limitation
One of the key limitations on how fast a battery can be charged is thermal heating. When charging a battery, its internal resistance generates heat, which must be dissipated into the environment to avoid damage. When charging occurs at high currents, the heat produced within the battery cannot be removed quickly enough, causing the temperature to rise rapidly. If the temperature exceeds safe limits, it can lead to damage or degradation of the battery's internal components, limiting the charging speed.
2. Mass Transfer Limitation
Mass transfer limitation occurs when the movement of lithium ions (Li+) through the electrolyte becomes a bottleneck during fast charging. Even with high surface area electrodes, the diffusion of Li+ ions is limited.
· Diffusion Limitation: Despite high surface area electrodes, the speed at which Li+ ions diffuse through the electrolyte restricts fast charging.
· Li+ Depletion Near the Anode: Li+ ions carry the current at the electrodes, leading to depletion near the anode, which limits further charging.
· Consequences of Exceeding Limits: If the current exceeds the diffusion limit, it can cause solvent decomposition, heating, and battery damage.
3. Impact of Battery Material on C Rating
Different battery materials exhibit varying charge rates due to these limitations. For instance, a typical NCM (Nickel Cobalt Manganese) lithium battery has a C rating of 1C, but its maximum C rate can reach around 10C, especially for 18650 batteries. On the other hand, a typical LiFePO4 (Lithium Iron Phosphate) lithium battery generally has a C rating of 1C, with a maximum C rate reaching 3C, particularly for LiFePO4 prismatic batteries.
These differences are largely due to the unique characteristics of the materials, including their internal resistance, ion diffusion properties, and overall structural design. Each type of battery material has its own optimal charging speed and limitations based on its inherent properties.
Overview of the Battery C Rate Chart
C Rating | Time |
30C | 2 mins |
20C | 3 mins |
10C | 6 mins |
5C | 12 mins |
2C | 30 mins |
1C | 1 hour |
0.5C or C/2 | 2 hours |
0.2C or C/5 | 5 hours |
0.1C or C/10 | 10 hours |
0.05C or C/20 | 20 hours |
Battery C Rating Chart
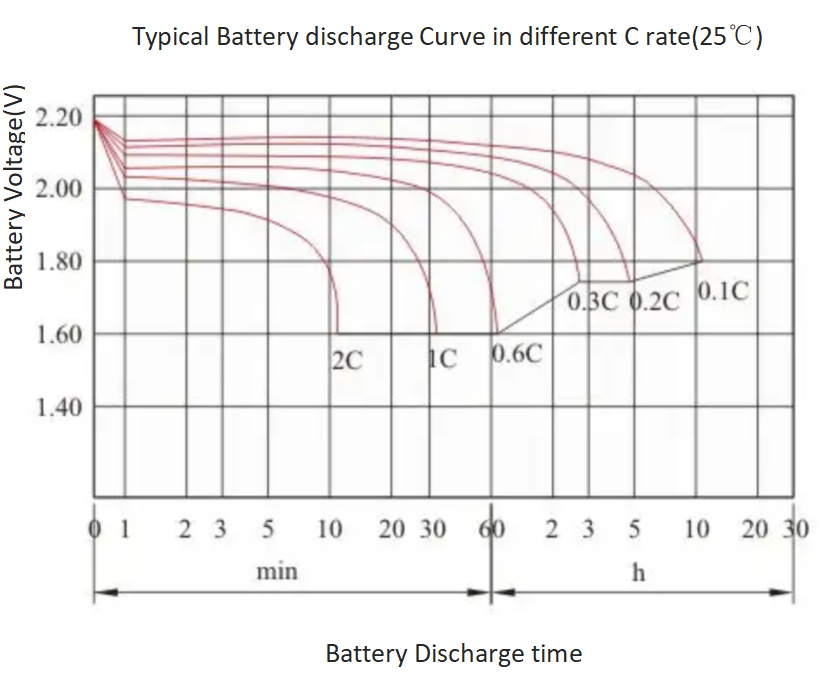
Battery Discharge Curve In Different Battery C Rating
How To Calculate The C Rating For The Battery?
Formula:
t = Time
Cr = C Rate
t = 1 / Cr (to view in hours)
t = 60 minutes / Cr (to view in minutes)
You can use the formula below to calculate a battery’s output current, power, and energy based on its C rating.
Er = Rated energy (Ah)
Cr = C Rate
I = Current of charge or discharge (Amps)
I = Cr * Er
Cr = I / Er
It can be known that the C rating of the battery is determined by the time required for charging or discharging。You can increase or decrease the C Rate and as a result this will affect the time it takes the battery to charge or discharge. The C Rate charge or discharge time changes in relation to the rating. 1C is equal to 60 minutes, 0.5C to 120 minutes and a 2C rating is equal to 30 minutes.
Let's take some examples:
0.5C Rate Example
· 6Ah battery
· 6Ah/1C=6Ah
· 6Ah×0.5C=3 Amps available
· 1/0.5C=2 hours
· 60 / 0.5C = 120 minutes
2C Rate Example
· 6Ah Battery
· 6Ah / 1=6Ah
· 2C x 6Ah = 12 Amps available
· 1 / 2C = 0.5 hours
· 60 / 2C = 30 minutes
30C Rate Example
· 6Ah Battery
· 6Ah / 1 = 6Ah
· 30C x 6Ah = 180 Amps available
· 60 / 30C = 2 minutes
Which Applications Require Batteries with a High C Rate?
With the development of technology, more application devices require high C rate batteries. They need to achieve a powerful energy burst in a short time, such as emergency start power supplies for drones, robots, and electric vehicles, etc.
If you need high-rate batteries to meet the requirements of your equipment, you can contact us. If you need a higher discharge rate of 50C or 80C, we will tailor a battery solution for your equipment. You can consult our professional engineers!
FAQs
How to find the C grade of a battery?
To find the C rating of a battery, check its label or datasheet. The C rating indicates the discharge rate, with 1C representing the one-hour rate. Different chemistries, like lithium, can tolerate higher C rates than others, such as lead acid. If not listed, contact the manufacturer for details.
What Are The Effects Of C Rating On Lithium-ion Batteries?
The C rating affects how quickly a battery can discharge energy. High C ratings are ideal for applications requiring rapid power, like motorcycle starters, while low C ratings suit applications with longer power needs, such as solar lights, which require longer discharge times.
What Is The C Rating Of My Battery?
The C rating of your battery is usually found on the label or datasheet. Lithium iron phosphate batteries typically have a 1C rate, NCM batteries 3C, and lead-acid batteries 0.05C. If not listed, contact the manufacturer.

